Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 ,
, -di(2-ethylhexyl)octanamide from nitric acid medium
-di(2-ethylhexyl)octanamide from nitric acid mediumTsutsui, Nao; Ban, Yasutoshi; Sagawa, Hiroshi; Ishii, Sho; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 35(6), p.439 - 449, 2017/08
Times Cited Count:5 Percentile:16.24(Chemistry, Multidisciplinary)Solvent extraction of uranium from a nitric acid medium was performed with  ,
, -di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as
-di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as 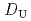 = 1.1
= 1.1![$$[rm NO^{-}_{3}]^{1.6}_{rm aq}[{rm DEHOA}]^{2}_{rm org}$$](/search/images/ERNVY2TNEBHE4XT1FV4V44ZTPVOV24ZRFY1H0X11LRZG0IDBOF4VW402OJWSARCFJBHUC5K3LZ3TE5K5PNOHE1JAN3ZGO5JE.gif) . Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation
. Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation 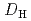 = 0.12
= 0.12![$$[rm H^{+}]^{0.76}_{rm aq}[{rm DEHOA_{rm Free}}]_{rm H}$$](/search/images/ERNVY2TNEBEF24ZLPVOV24ZQFY1TM5K5PNOHE1JAMFYX0W11LRZG0ICEIVEE4QK5PNOHE1JAIZZGKZL3PVOV4402OJWSASD3EQ.gif) was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm
was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm (M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
(M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
 -dialkylamides using multistage mixer-settler extractors
-dialkylamides using multistage mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Tsubata, Yasuhiro; Matsumura, Tatsuro
Procedia Chemistry, 21, p.156 - 161, 2016/12
Times Cited Count:5 Percentile:94.32(Chemistry, Inorganic & Nuclear)A continuous counter-current experiment was carried out to demonstrate the validity of a process using  -dialkylamides for recovering U and Pu. This process consisted of two cycles, and the 1st cycle and the 2nd cycle employed
-dialkylamides for recovering U and Pu. This process consisted of two cycles, and the 1st cycle and the 2nd cycle employed  -di(2-ethylhexyl)-2,2-dimethylpropanamide and
-di(2-ethylhexyl)-2,2-dimethylpropanamide and  -di(2-ethylhexyl)butanamide as extractants, respectively. The feed solution for the 1st cycle was 5.1 mol/dm
-di(2-ethylhexyl)butanamide as extractants, respectively. The feed solution for the 1st cycle was 5.1 mol/dm (M) nitric acid containing 0.92 M U, 1.6 mM Pu, and 0.6 mM Np. The raffinate collected in the 1st cycle was used as the feed for the 2nd cycle. The ratios of U recovered in the U fraction and U-Pu fraction were 99.1% and 0.8%, respectively. The ratio of Pu recovered in the U-Pu fraction was 99.7%. The concentration ratio of U with respect to Pu in the U-Pu fraction was 9, and this indicated that Pu was not isolated. The decontamination factor of U with respect to Pu in the U fraction was obtained as 4.5
(M) nitric acid containing 0.92 M U, 1.6 mM Pu, and 0.6 mM Np. The raffinate collected in the 1st cycle was used as the feed for the 2nd cycle. The ratios of U recovered in the U fraction and U-Pu fraction were 99.1% and 0.8%, respectively. The ratio of Pu recovered in the U-Pu fraction was 99.7%. The concentration ratio of U with respect to Pu in the U-Pu fraction was 9, and this indicated that Pu was not isolated. The decontamination factor of U with respect to Pu in the U fraction was obtained as 4.5 10
10 . These results supported the validity of the proposed process.
. These results supported the validity of the proposed process.
Ban, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Matsumura, Tatsuro
Proceedings of 5th International Conference on Asian Nuclear Prospects 2016 (ANUP 2016) (CD-ROM), 2 Pages, 2016/10
A continuous counter-current experiment was conducted using mixer-settler extractors installed in a hot cell.  -Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) was used as the extractant for U. The experimental results showed that DEHDMPA can effectively extract U from nitric acid, and the recovery of U in the U fraction was obtained as 99.6%.
-Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) was used as the extractant for U. The experimental results showed that DEHDMPA can effectively extract U from nitric acid, and the recovery of U in the U fraction was obtained as 99.6%.
 ,
, -di(2-ethylhexyl)octanamide toward uranium from nitric acid
-di(2-ethylhexyl)octanamide toward uranium from nitric acidTsutsui, Nao; Ban, Yasutoshi; Sagawa, Hiroshi; Ishii, Sho; Matsumura, Tatsuro
Proceedings of 5th International Conference on Asian Nuclear Prospects 2016 (ANUP 2016) (CD-ROM), 2 Pages, 2016/10
Extraction properties of  ,
, -di(2-ethylhexyl)octanamide (DEHOA) toward uranium from nitric acid were studied using single-stage batch method. The results revealed that DEHOA effectively extracted uranium when the nitrate ion concentration was 2.0-3.0 mol/dm
-di(2-ethylhexyl)octanamide (DEHOA) toward uranium from nitric acid were studied using single-stage batch method. The results revealed that DEHOA effectively extracted uranium when the nitrate ion concentration was 2.0-3.0 mol/dm .
.
 -dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractors
-dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 34(1), p.37 - 47, 2016/01
Times Cited Count:9 Percentile:29.47(Chemistry, Multidisciplinary)The extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and  -di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was
-di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was  3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
 -dialkylamides as extractants for U and Pu by continuous counter-current extractors
-dialkylamides as extractants for U and Pu by continuous counter-current extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Tsutsui, Nao; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1147 - 1152, 2015/09
Extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA),
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA),  -di(2-ethylhexyl)butanamide (DEHBA), and some of their degradation products for the metal elements Zr, Mo, Ru, Rh, and Pd were studied using a single-stage batch method, and the results showed that the degradation products barely extracted these metal elements. Furthermore, separation performance of DEHDMPA and DEHBA for U and Pu in a continuous counter-current process was evaluated using a calculation code, and it was confirmed that the calculated values of U concentration in the U fraction and U and Pu concentrations in the U-Pu fraction were similar to those measured experimentally. These results supported the applicability of DEHDMPA and DEHBA as extractants for separation processes and the validity of the calculation code for estimating the separation performance of the process.
-di(2-ethylhexyl)butanamide (DEHBA), and some of their degradation products for the metal elements Zr, Mo, Ru, Rh, and Pd were studied using a single-stage batch method, and the results showed that the degradation products barely extracted these metal elements. Furthermore, separation performance of DEHDMPA and DEHBA for U and Pu in a continuous counter-current process was evaluated using a calculation code, and it was confirmed that the calculated values of U concentration in the U fraction and U and Pu concentrations in the U-Pu fraction were similar to those measured experimentally. These results supported the applicability of DEHDMPA and DEHBA as extractants for separation processes and the validity of the calculation code for estimating the separation performance of the process.
Sugo, Yumi; Sasaki, Yuji; Tachimori, Shoichi
Radiochimica Acta, 90(3), p.161 - 165, 2002/04
Times Cited Count:153 Percentile:99.24(Chemistry, Inorganic & Nuclear)no abstracts in English
Tachimori, Shoichi; Suzuki, Shinichi; Sasaki, Yuji
Nihon Genshiryoku Gakkai-Shi, 43(12), p.1235 - 1241, 2001/12
Times Cited Count:29 Percentile:87.17(Nuclear Science & Technology)no abstracts in English
Suzuki, Shinichi; ; Tachimori, Shoichi; *
Journal of Radioanalytical and Nuclear Chemistry, 239(2), p.377 - 380, 1999/00
Times Cited Count:10 Percentile:60.66(Chemistry, Analytical)no abstracts in English
; Tachimori, Shoichi
Bulletin of the Chemical Society of Japan, 70(1), p.135 - 142, 1997/00
Times Cited Count:2 Percentile:19.46(Chemistry, Multidisciplinary)no abstracts in English
Tachimori, Shoichi; Yaita, Tsuyoshi; Suzuki, Shinichi
Proc. of Workshop on Long-Lived Radionuclide Chemistry in Nuclear Waste Treatment, p.179 - 188, 1997/00
no abstracts in English
 ,
, -dialkylamides-nitric acid systems by turbidity measurement, 2; The Correlation between turbidity and the distribution ratio of uranium
-dialkylamides-nitric acid systems by turbidity measurement, 2; The Correlation between turbidity and the distribution ratio of uraniumTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Urabe, Shunichi; Matsumura, Tatsuro
no journal, ,
The turbidity of the agitated solutions of  ,
, -di(2-ethylhexyl)-butanamide (DEHBA) in dodecane and uranyl nitrate in 3 M HNO
-di(2-ethylhexyl)-butanamide (DEHBA) in dodecane and uranyl nitrate in 3 M HNO was measured for 30 min. The variation of the distribution ratio of uranium after stopping the agitation was also measured, and the correlation between the turbidity and the distribution ratio was studied. The turbidity and the distribution ratio of uranium at 3 min after stopping the agitation was more than 1000 FTU and 1.7, respectively, when the initial uranium concentration was 700 mM. The turbidity at 6 min decreased to 363 FTU, while the distribution rate increased to 2.2. Although the turbidity after 6 min decreased gradually, the distribution ratio barely changed. The correlation between the turbidity and the distribution ratio is discussed.
was measured for 30 min. The variation of the distribution ratio of uranium after stopping the agitation was also measured, and the correlation between the turbidity and the distribution ratio was studied. The turbidity and the distribution ratio of uranium at 3 min after stopping the agitation was more than 1000 FTU and 1.7, respectively, when the initial uranium concentration was 700 mM. The turbidity at 6 min decreased to 363 FTU, while the distribution rate increased to 2.2. Although the turbidity after 6 min decreased gradually, the distribution ratio barely changed. The correlation between the turbidity and the distribution ratio is discussed.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka
no journal, ,
The new reprocessing and minor actinide (MA) separation processes using innovative extractants in accord with CHON principle has been developed in Japan Atomic Energy Agency aimed for reduction of radioactive wastes from nuclear fuel cycle. The new nonorganophosphorus extractants which have appropriate extraction behaviors for each separation steps were developed. Continuous counter-current experiment of each solvent extraction process with uranium, plutonium and tracers of minor actinides were carried out. The experimental results showed that the separation performance of the solvent extraction processes were demonstrated successfully.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; et al.
no journal, ,
PUREX process was established for industrial scale reprocessing plant. TRUEX and the 4 group separation were developed for partitioning of minor actinides from HLW, and demonstrated using genuine HLW. Although the extractants for the processes have excellent performance, the molecules contain phosphorus which could be cause for the secondary waste from the solvent extraction processes. To minimize the radioactive waste, we have conducted research and development of the new reprocessing and MA separation processes using innovative extractants in accord with CHON principle. The extractants for reprocessing process are monoamides as alternative extractants for TBP. For An(III)+RE recovery process, we developed TDdDGA. HONTA and ADAAM were developed for An(III)/RE separation process and Am/Cm separation process respectively. The separation performances of the flowsheets were evaluated by continuous extraction tests using simulated and genuine spent fuel and high level liquid waste.
Ban, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Tsubata, Yasuhiro; Matsumura, Tatsuro
no journal, ,
no abstracts in English
Iwamoto, Toshihiro; Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Naruse, Atsuki*; Tsukahara, Takehiko*
no journal, ,
The sludge generated production of nuclear fuel contained uranium has been storage. The sludge is immersed in some kinds of solution. After immersion, uranium is recovered from the solution. Cerium extractive tests using thermoresponsive polymer was carried out on two kinds of extractants. C14-BAMA was found to be superior, and we plan to conduct a uranium study on this extractant.
Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Naruse, Atsuki*; Tsukahara, Takehiko*
no journal, ,
The sludge contained uranium generated production of nuclear fuel has been storage. The sludge is immersed in some kinds of solution. After immersion, uranium is recovered from the solution. Solvent extraction method, extraction chromatography and gelling extraction method were conducted on uranyl nitrate solution using monoamide extractant to compare on amount of waste and running cost on each methods. The gelling extraction method was superior to other two methods.